|

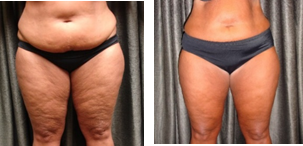
Verjú LLLT by Erchonia, FDA Cleared for circumferential reduction of the waist, hips and thighs and temporary reduction in the appearance of cellulite will be featured on the programme at the Aesthetic and Anti-Aging Medicine Congress 2018. The Verjú laser produces a low-level output that has no thermal effect on the body’s tissue. Its unique technology does not kill or damage fat cells, but instead creates a transitory pore in each cell for the fat to leak out, which is then processed naturally through the lymphatic system, leaving the cell intact for important endocrine function.
The 510(k) is a premarketing submission to the FDA that demonstrates that the device marketed is safe and effective. The premarket approval for the 510(k) is the most rigorous type of device marketing application accepted by the FDA. The Verjú Laser System obtained its 1st 510(k) clearance in 2012.
Verjú Study Results – NO lifestyle changes – Published in American Journal of Cosmetic Surgery for Body Contouring and Lasers in Surgery in Medicine for Appearance of Cellulite. There were no adverse events in either study.
A world leading Dermatologist and Cosmetic Surgeon, Michael H. Gold, M.D., FAAD, will present Thursday 5th April @ 10:00AM. Dr. Gold’s message is ‘A Better You’!
Simon Ramshaw, Managing Director of Erchonia Lasers LTD. comments, “Having an expert such as Dr. Gold recognize the quality of our research and the clinical utility of Erchonia’s Verjú Laser
System is truly an honor. Quality clinical research is pivotal for our overall success. Erchonia’s commitment to research stands out in aesthetic medicine.”
Founded in 1996, Erchonia Corporation is the world leader in low-level laser technology. The company created the low-level laser category in 2002 when it received an FDA 510(k) market clearance for low-level lasers. Erchonia was the first company to receive this FDA 510(k) distinction. For more information, visit www.erchonia.com or call 888-242-0571.
For additional information, image and interview requests, contact Erchonia Lasers Ltd at +44(0)1491 821135.
Related Links https://www.Erchonia.com |
