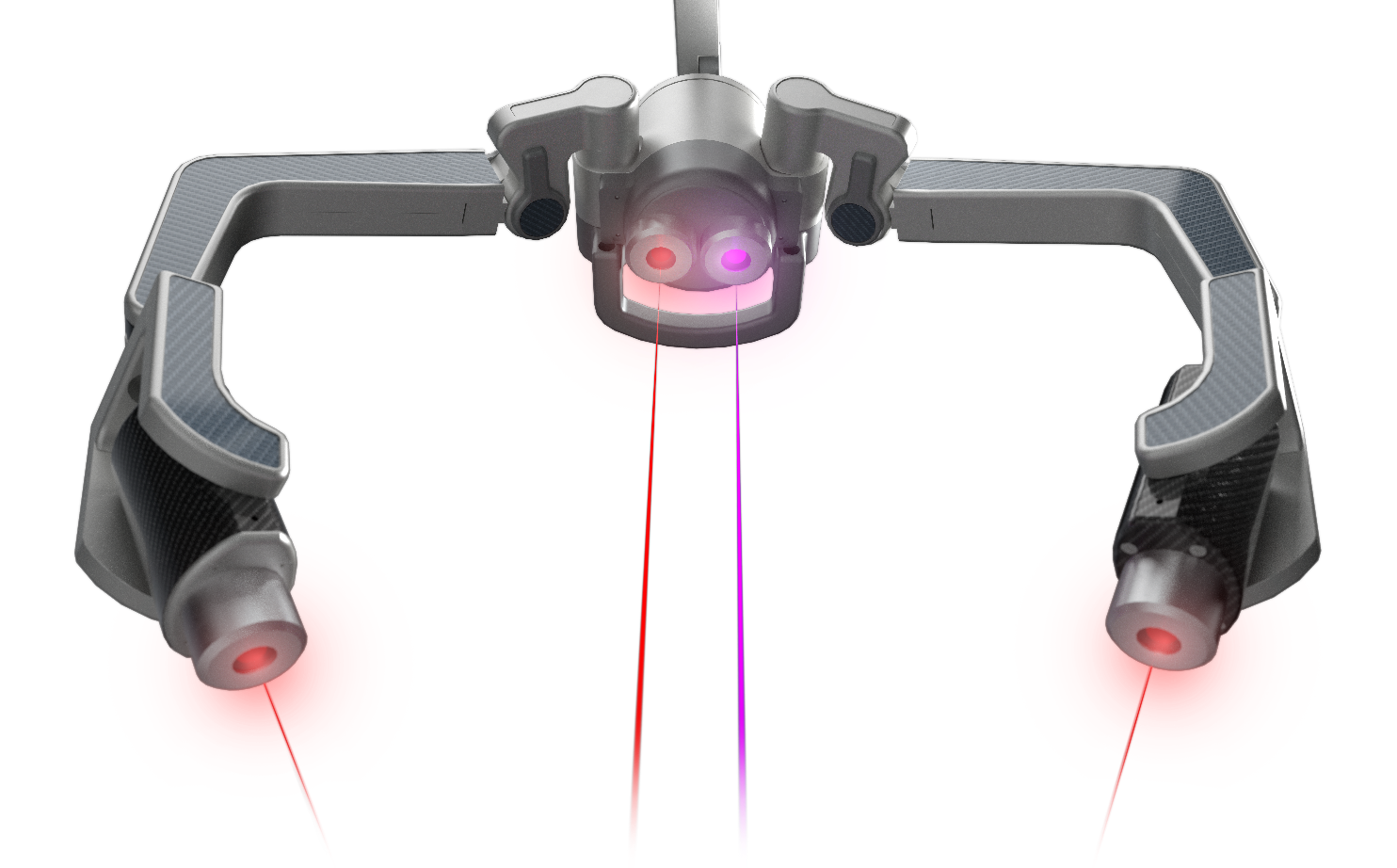
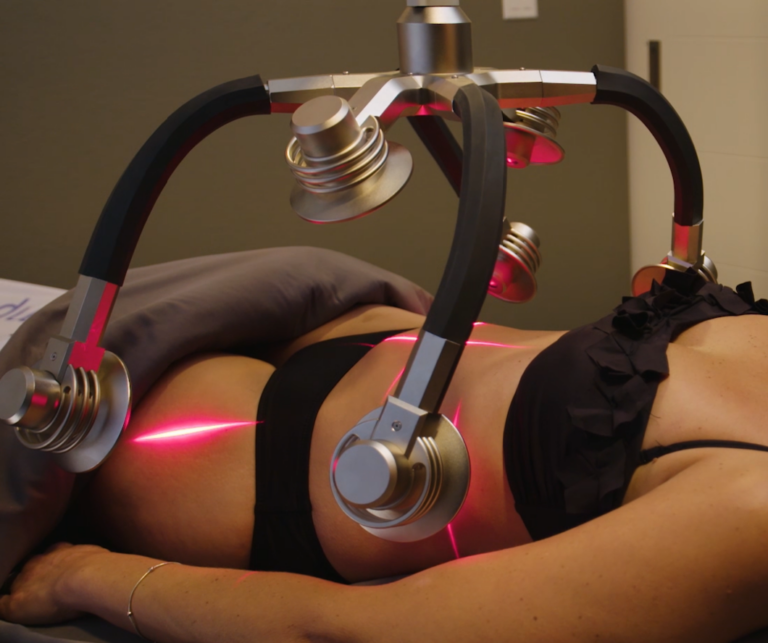
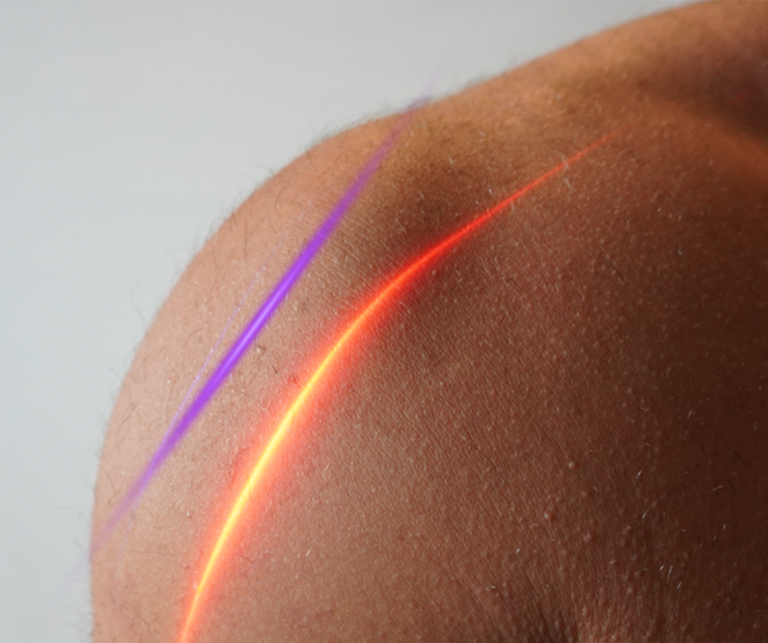
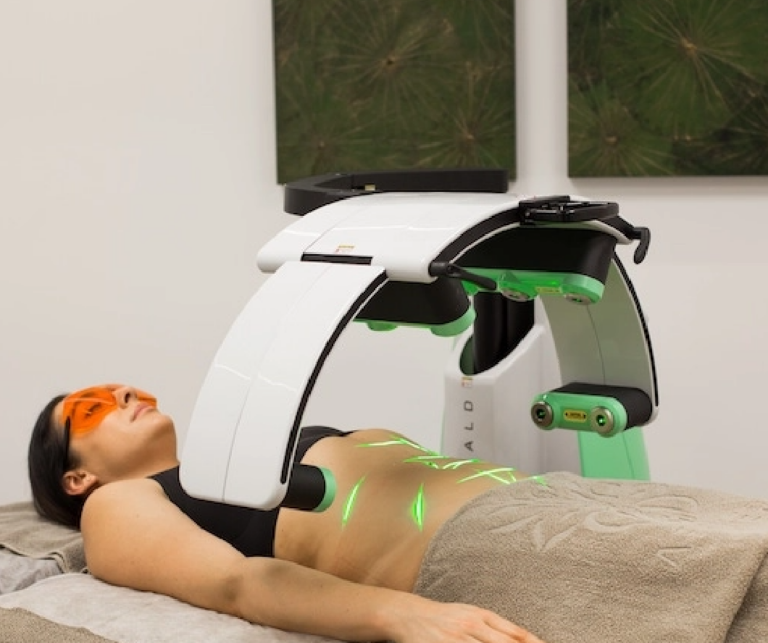
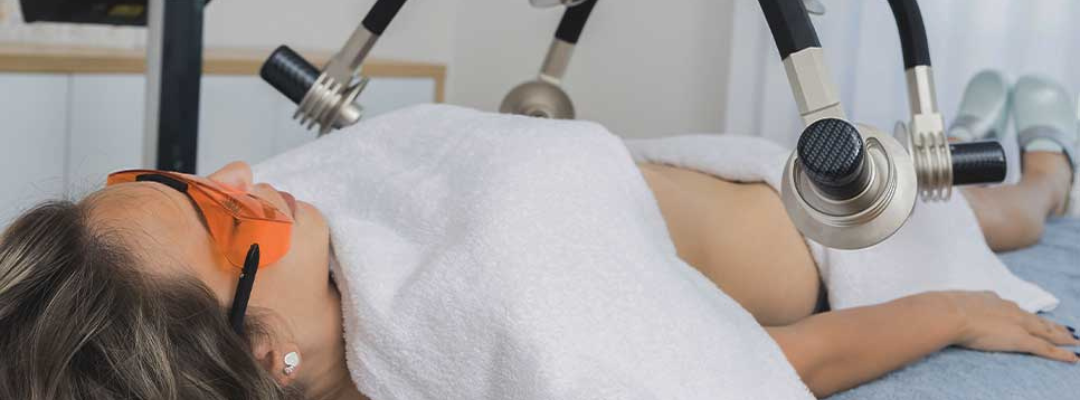
Currently, there are many acne treatments available in the form of pills, lotions, tonics, chemicals, and more, but Erchonia has developed a new solution with the EVRL LASER. This FDA market cleared laser targets the actual type of bacteria that is responsible for causing acne, rather than targeting the skin. This treatment does not lead to irritated skin cells because it attacks the cause of acne at the root. Rather than spending many months or years on powerful antibiotics, creams, and more, patients can now attach their acne with painless low-level laser treatments. Cold lasers are currently undergoing studies for new indications for use. Though it is a relatively new field of medicine, Erchonia’s commitment to low-level laser treatment has made great advancements for laser technology. The future of medical lasers is certainly bright.