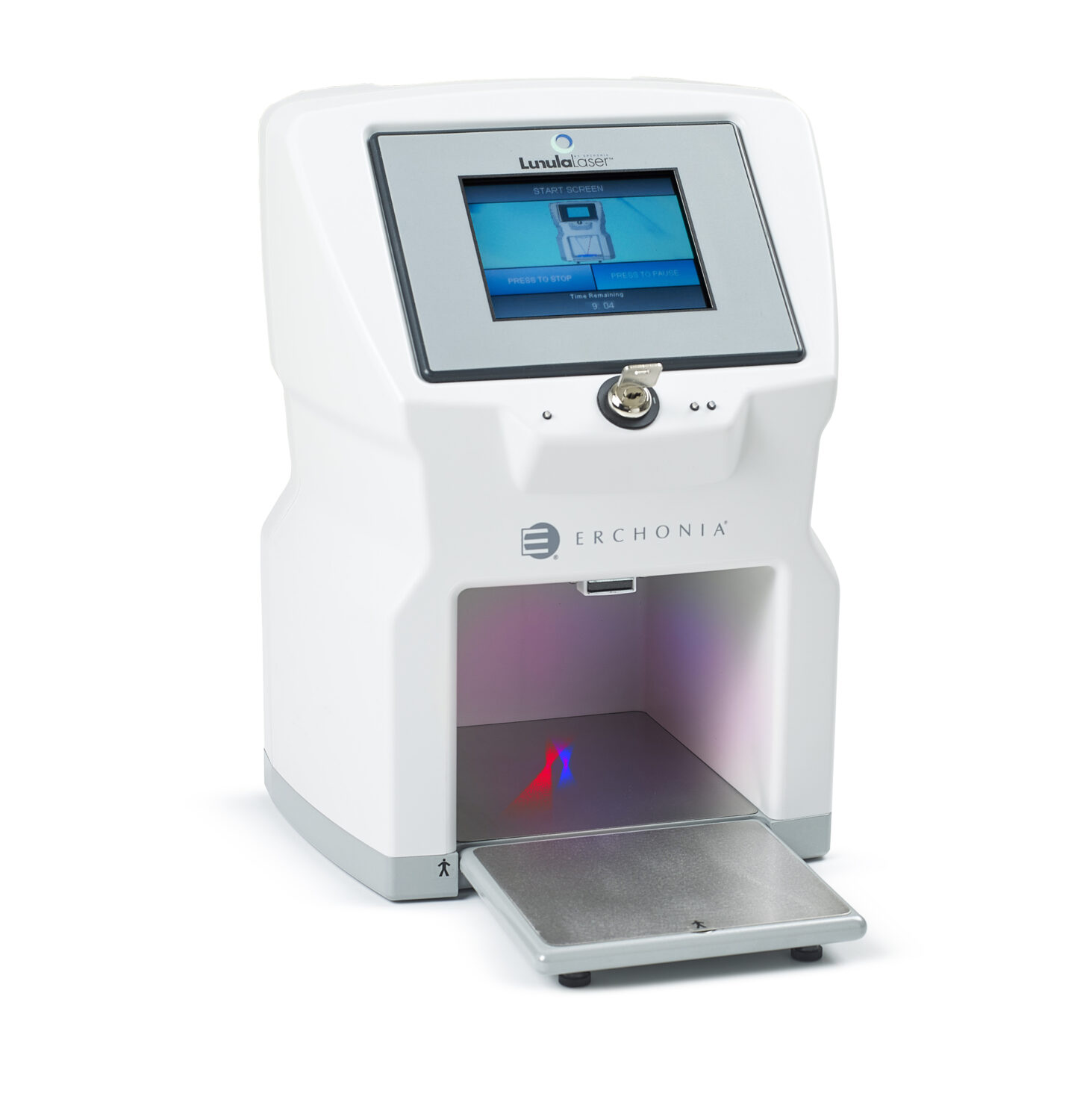
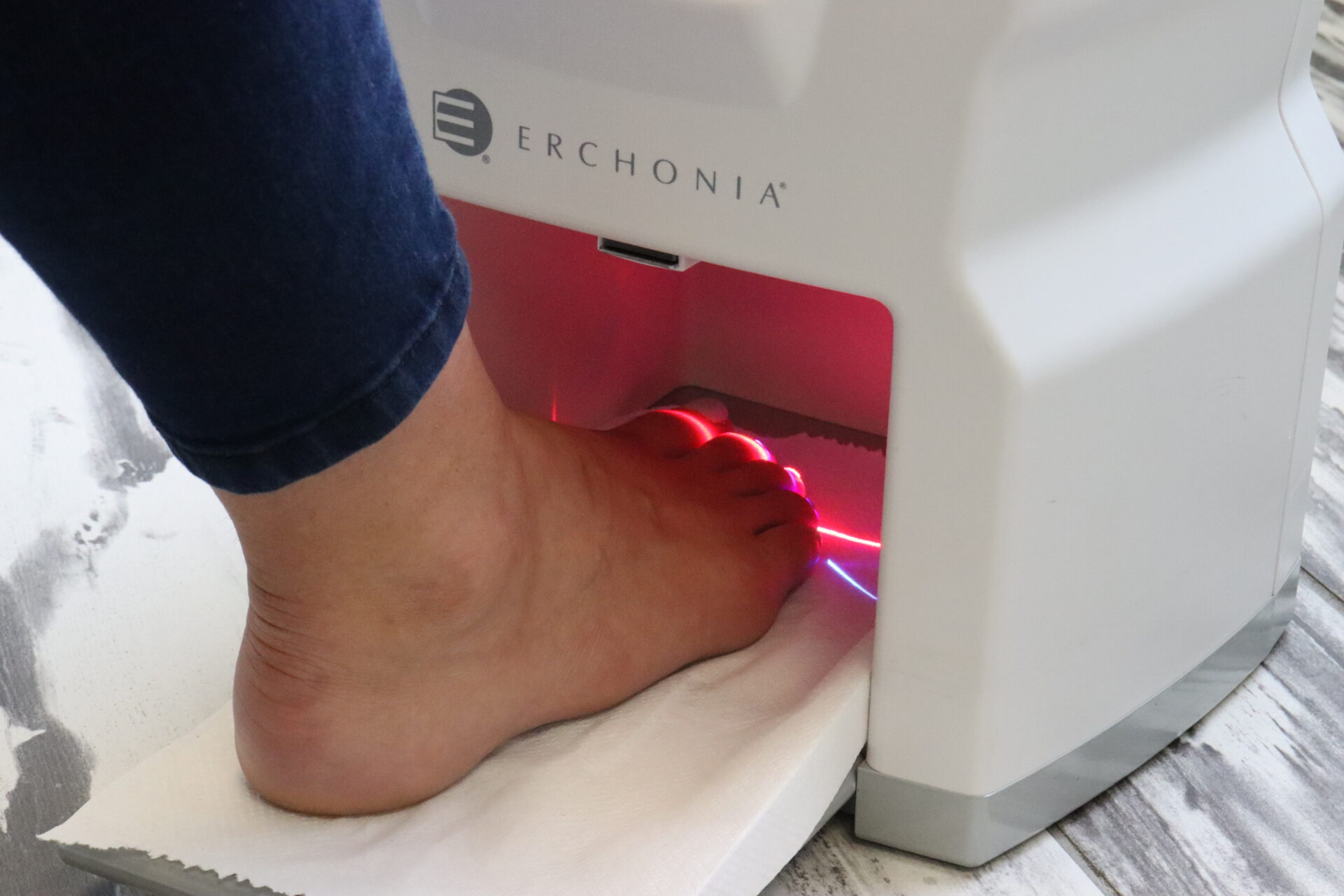
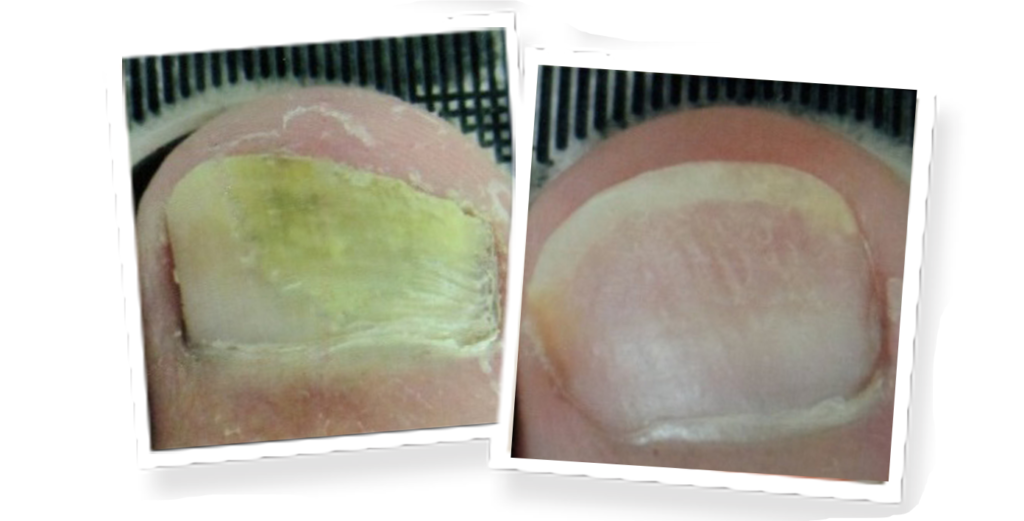
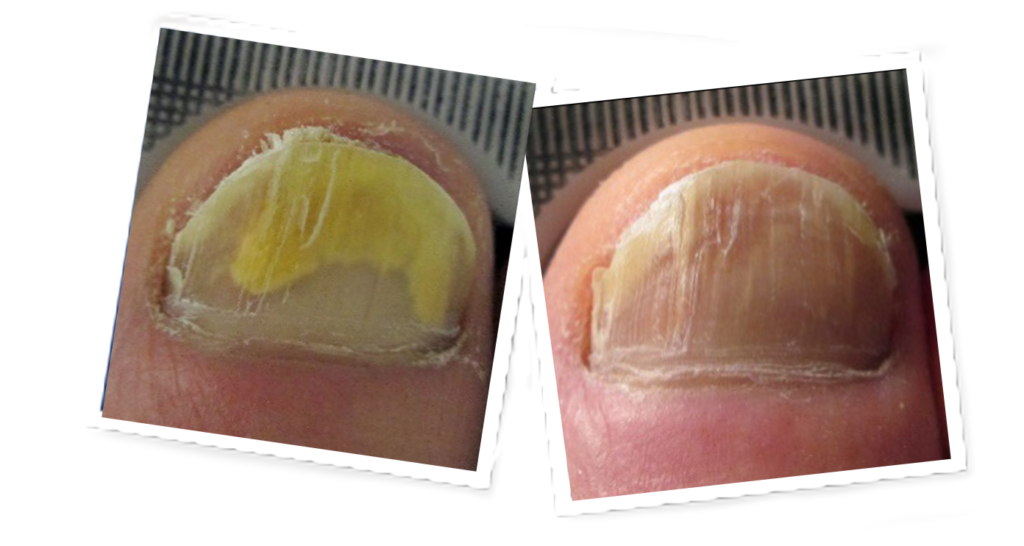
Lunula Laser®
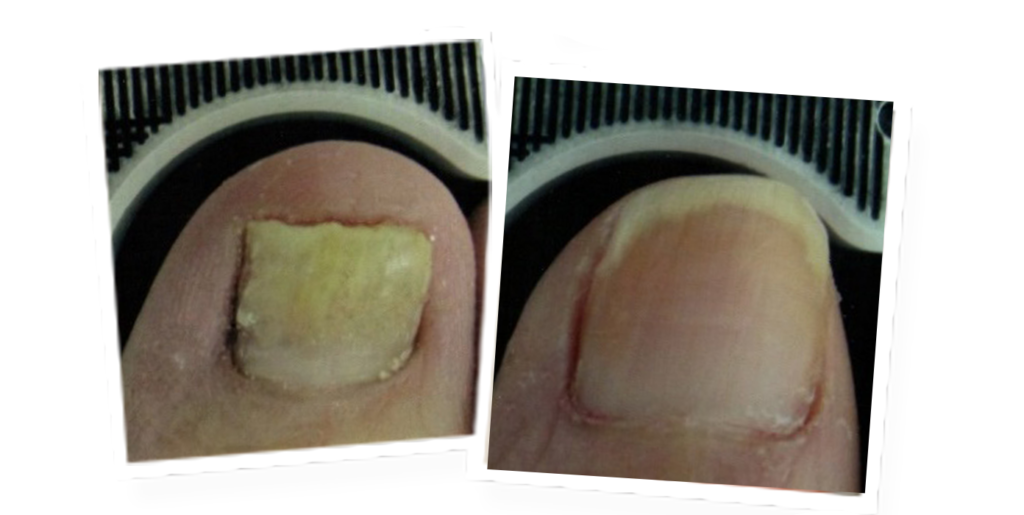
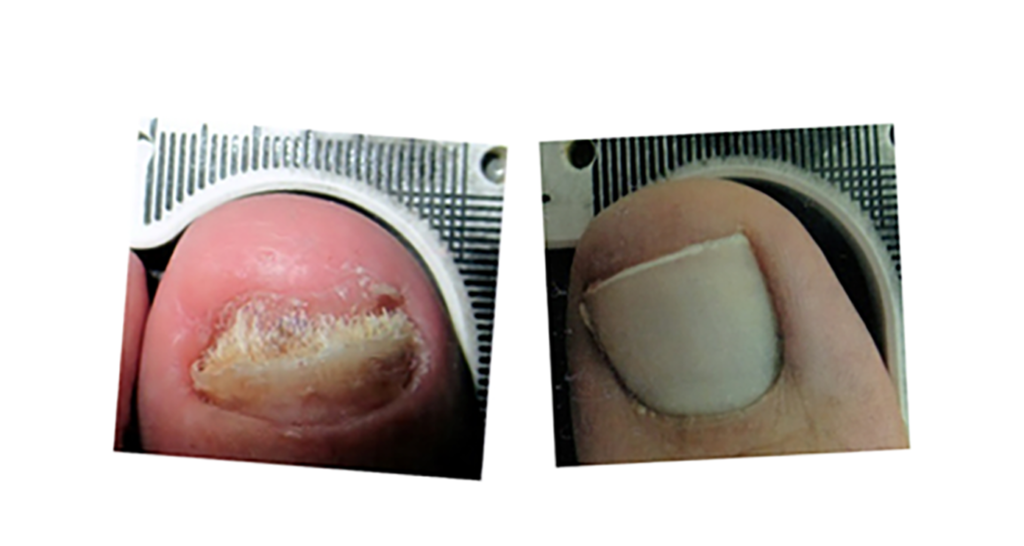
The revolutionary Lunula laser brings new hope to people suffering from onychomycosis. Safe and effective, Lunula Laser is the first and only non-thermal laser FDA cleared for increasing new clear nail growth in individuals with onychomycosis. The innovative Lunula foot fungus laser poses none of the risks and harmful side effects of oral anti-fungal medications and is painless, unlike other laser therapies.
The Lunula advantage:
- No heat, pain, or downtime
- 6.1 mm new clear nail growth at 6 months
- 12 minute treatments
- Treats all 5 toes at once
- FDA cleared
Indications for Use: – K153164
The Lunula Laser™ device is indicated for use for the temporary increase of clear nail in patients with onychomycosis
(e.g., dermatophytes Trichophyton rubrum and T. mentagrophytes, and/or yeasts Candida albicans, etc.)
*Device intended for Medical Professionals
*Prescription Use (Part 21 CFR 801 Subpart D)