Back pain is a common condition experienced around the world, with 80% of adults in Europe and the U.S. experiencing some type of back pain and up to 20% developing chronic back pain.
If you have chronic back pain, you have alternatives to more traditional treatments. Back pain treatment options include non-invasive methods that can help you find relief.
What Is Chronic Back Pain?
Chronic back pain lasts for at least three months. This pain may persist in some people for years. Chronic pain can cause what doctors call the “terrible triad,” a series of symptoms including sadness, sleeplessness and suffering. Many adults with chronic low back pain experience a decrease in activity levels or an inability to work due to their pain and related symptoms.
Causes of chronic pain in the back vary among individuals and can include injuries, infections, tumors, arthritis, nerve problems or vertebrae issues. The origin of the pain may inform your doctor of the best specialist to visit. For instance, if you have problems with the vertebrae, you may get a recommendation to see an orthopedist. However, if your nerves cause pain, you might go to a neurologist.
Alternative Treatments for Chronic Back Pain
Even with an evaluation and treatment from a doctor with traditional medicine, some people continue to suffer from chronic low back pain. Finding alternative treatment methods to ease suffering is vital to their health and well-being. The good news about many non-invasive pain-relieving methods is that you can combine them to find the best means of easing discomfort. Here are some common alternative therapies to take note of:
1. Massage or Spinal Manipulation
Massage therapy feels good and could help with pain relief. It may benefit those who have chronic back pain caused by injuries, fibromyalgia or rheumatoid arthritis.
Don’t confuse massage therapy, which focuses on pressing the muscles, with spinal manipulation, which is a common treatment given by chiropractors. Studies back up the use of spinal manipulation for many types of chronic back pain. For instance, 85% of patients with spondylolisthesis — a condition where a vertebra slips onto the one under it — as the cause of their back pain showed a reduction in discomfort.
Additionally, 90% of those with a diagnosis of sacroiliac syndrome, a condition affecting the sacroiliac joints near the low back, reported less pain from spinal manipulation.
2. Biofeedback
Biofeedback involves using relaxation exercises to create changes in measured biological functions. The patient has monitoring devices connected to them to give data about blood pressure, muscle tension, breathing and heart rates, brain waves and skin temperature. By practicing meditation or similar exercises, the patient learns to take control of automatic bodily functions. With greater control, patients using biofeedback can reduce stress and pain associated with excessive tension. Some studies support biofeedback for improving pain conditions, such as fibromyalgia and headaches.
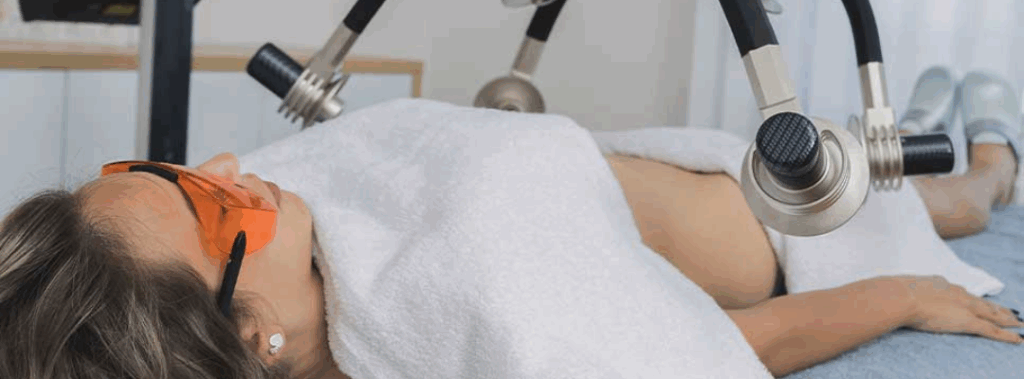
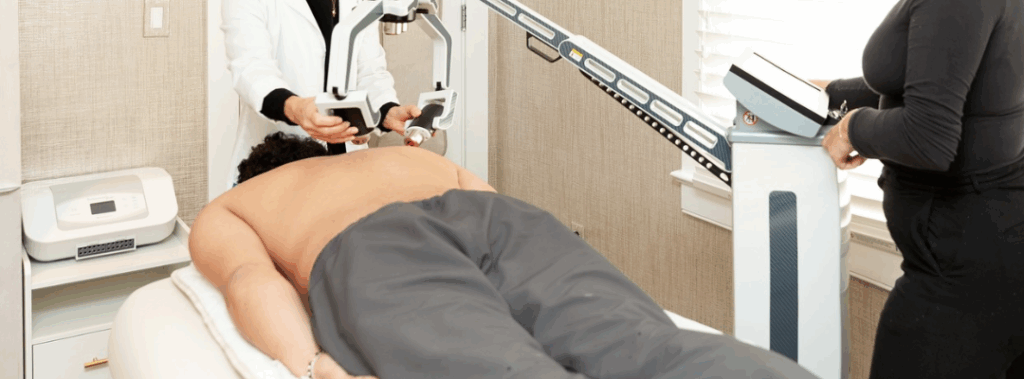
3. Laser Treatment
Chronic back pain could ease through laser therapy. A study of the FX 635 low-level laser showed a 58% reduction in back pain two months after treatment. This laser uses an effective low-level treatment that reduces inflammation. Because it has such a focused beam, the doctor can target the laser directly at the affected spot. Additionally, you can use laser therapy in addition to other forms of non-invasive treatments for back pain. Find a provider near you!
4. Yoga
The gentle stretches and meditation of yoga make it ideal for those with chronic back pain. The meditative aspect helps reduce stress while the stretching and movement can decrease pain. It has enough evidence to support its use for fibromyalgia, back pain and arthritis that some doctors prescribe it as part of a treatment regime.
5. Relaxation Therapy or Meditation
Relaxation therapy can help reduce muscle tension and stress and meet your psychological needs when dealing with back pain. Some people with chronic back pain may experience irritability, depression or frustration. Therefore, meditation and other relaxation techniques that address the psychological effects can help your overall health.
6. Lifestyle Modifications
Change your lifestyle to meet your physical limitations. Trying to exceed your physical limits could cause strain and additional pain. Don’t give up regular movement or a healthy lifestyle, but avoid activities that cause significant pain. If you need assistance finding the right balance of activity in your life, talk to your doctor or a physical therapist.
7. Physical Therapy
Physical therapy differs from regular exercise. During appointments with a physical therapist, you will learn movements and exercises to strengthen and stretch specific areas of your back that need help. Types of work you may do with a physical therapist include core training, posture exercises, pain limitation testing, flexibility training and aerobics.
Everyone has unique physical therapy needs, so you cannot simply read about exercises or attend a class. Ask your insurance if you need a referral from your physician to make an appointment with a physical therapist.
8. Transcutaneous Electrical Nerve Stimulation (TENS)
TENS is a non-invasive technique that sends low-voltage electric currents through the skin to ease pain. In theory, the electricity passing through your skin blocks some of the pain signals from reaching the spinal cord and brain. Additionally, it could promote production of the body’s natural painkillers, like endorphins.
Despite conflicting scientific evidence for the effectiveness of using a TENS unit, patients often report some pain relief while using it. Plus, it does not have side effects and is safe for most people to use. Some doctors consider it safe enough to use with easing labor pains without harming the mother or child. However, those in early pregnancy or people with pacemakers or conditions like epilepsy should not use TENS units.
9. Acupuncture
Acupuncture could produce results for those suffering from chronic pain, including back pain. Researchers combined information from 29 previous studies that included a total of 18,000 patients. Overall, the results showed an average of 50% of participants in all the studies received pain relief from acupuncture.
When starting with acupuncture, you may want to schedule weekly visits with the provider. Based on how your pain responds to the procedure, you could spread out the appointments to obtain maximum relief.
Find Out More About Laser Treatment for Back Pain
While you can get most of the above treatments outside of doctors’ offices, you need a physician to administer laser therapy. Ask your doctor if they offer laser treatment for back pain. If your current doctor doesn’t offer laser treatment, request it or search for a physician in your area that provides back pain therapy with lasers.
If you want to integrate any of the above non-invasive treatment options for chronic back pain into your plan, talk to your doctor about which will work best for you. As a patient, you have several options for back pain, including laser treatment and other alternative methods. Exercise your right to get the best, most comprehensive care possible by having a conversation with your doctor about these non-invasive methods of pain relief.
Find a Provider Become a Provider